Toxicity reference values (TRVs)
In response to a request by the Ministries of Health and environment, the Agency set up an expert group dedicated to TRVs in 2008. Its aim is to establish TRVs for substances of interest and introduce the procedures for their support, the framework for their dissemination and use, and how they are to be monitored over time. This group also aims to develop and enhance methodological knowledge relating to TRVs.
Anses derives several types of TRVs:
- external TRVs, specific to an exposure route (oral, inhalation, dermal);
- internal TRVs, expressed as concentrations in a biological matrix of the human body (blood, urine, etc.).
The latter are accompanied by reference values based on the results of biomonitoring studies of the general population, known as population biomonitoring values (PBVs). These do not allow interpretation in terms of health risk.
How are TRVs developed?
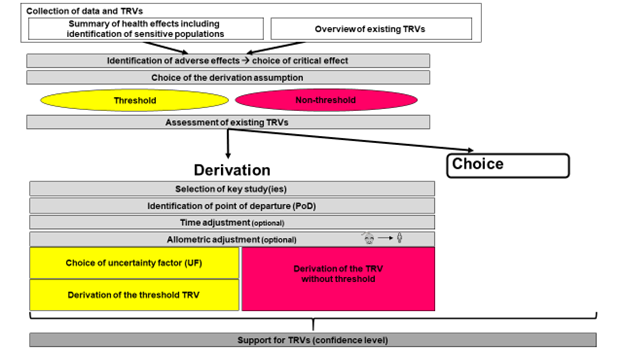
By definition, TRVs are established to protect the entire population, including sensitive populations (e.g. children, the elderly, etc.), from adverse effects induced by the chemical agent.
Derivation of external TRVs involves the following steps, as outlined in the different stages shown in the figure.
To derive internal TRVs, the first step is to identify a relevant biological indicator of exposure (BIE, or exposure biomarker) (the chemical of interest or one of its metabolites in the selected biological matrix). Several approaches can then be used: derivation based on a relationship between BIE concentrations and health effects, or, if such a relationship is not available, derivation based on an external TRV or an external point of departure identified from one or more key studies.
The report entitled “Derivation and selection of reference values” (2025, report in French only) sets out the main methodological elements useful for establishing the various reference values, including external and internal TRVs (with or without a threshold) and PBVs. It represents a consolidation of several guides, harmonizing methods across reference values and updating them to reflect new scientific developments. It is intended for ANSES staff and experts, as well as for decision-makers and users of reference values (government services, consultancy firms, industry, occupational physicians, prevention specialists, etc.).
After a review of existing methods for considering mixtures (report in French), Anses proposed a tiered approach to develop TRVs for a mixture of substances, considering either the whole mixture or each substances of the mixture. This approach was applied to the example of the BTEX mixture (benzene, toluene, ethylbenzene, xylenes) (report in French).
The work conducted by the Agency
So far, about a hundred substances TRVs have been formulated by the Agency for about sixty chemical substances.
> See page "List of toxicity reference values (TRVs) established by ANSES"
ANSES has also compiled a database of around 500 TRVs that it has chosen to use for its own expert appraisal work. Making this database available facilitates access to the TRVs for all users, in particular the various public and private partners (consultancies, regional directorates for the environment, land planning & housing, regional health agencies, INERIS, etc.).
All the TRVs established or selected by ANSES in the context of collective expert appraisals carried out within the Agency are available from the Excel table below. This table lists:
- the substance and its CAS number;
- the year the TRV was established and by whom;
- the TRV type (short, mid and long term);
- the route of exposure;
- the targeted population;
- the value and unit of the TRV;
- the expert appraisal during which the TRV was established or selected, as well as its year of publication, with a link to the report in pdf format.
> Download the table of TRVs established or selected by ANSES (Excel) (in French)
The European reference values for active plant protection and biocidal substances are available from the following databases:
- active plant protection substances:
- active biocidal substances: Database managed by ECHA
The reports describing the internal guide values derived in the European project HBM4EU (HBM-GV) for the general population or the occupational population are available via the following link: hbm4eu.eu/deliverables (Work Package 5: Translation of results into policy).
Which substances is ANSES currently examining?
The substances for which ANSES has been asked to develop TRVs, either through formal requests or internal requests (for risk assessment or related to work on indoor air quality guidelines - IAQGs) or occupational exposure limits to chemicals - OELs)) or for establishing the development method, have been identified and included in the 2024-25 work programme:
- Lithium (intern et respiratory TRVs)
- Perfluorinated compounds (long-term oral TRVs)
- Ultrafine particles (PUF) (short- and long-term respiratory TRVs)