New genomic techniques (NGTs) : ANSES calls for appropriate regulations
New genomic techniques (NGTs) offer a vast range of applications, especially in the breeding of cultivated plants. Similar to genetically modified organisms (GMOs), but nevertheless distinct from transgenic plants, these applications require careful consideration with a view to their possible arrival on the European market. Within the scope of its missions, ANSES conducted an expert appraisal of the challenges associated with these NGTs in order to provide the authorities and stakeholders with insights for the current discussions on changes to the European GMO framework. The Agency proposed adapting the assessment of these plants on a case-by-case basis, using a graduated approach, and recommended a comprehensive monitoring scheme. In addition to the health challenges, ANSES also identified various socio-economic motivations and concerns associated with NGTs in agriculture, and called for future decisions to be based on a democratic debate that goes beyond the risks and considers all the issues at stake.
Anticipate the development of applications for the CRISPR-Cas system
Since GMOs first began to be regulated in the European Union in 2001, several techniques for genetically modifying plants – known as NGTs – have been developed. In particular, the CRISPR-Cas system can be used like a pair of molecular scissors to modify a genetic sequence in a precise and targeted way. Site-directed mutagenesis, i.e. limited modification of the plant genome at the sites chosen by the breeder, predominates among the most popular uses of NGTs in agricultural plant breeding.
From GMOs to plants derived from NGTs, what changes have been seen?
Transgenesis is the technique historically used to create GMOs. It consists in introducing one or more genes from one species into the genome of another organism, with the aim of acquiring new characteristics. Unlike transgenesis, certain NGTs (site-directed mutagenesis) do not require the addition of genes from species with which crossbreeding would have been impossible in nature. The organisms derived from these NGTs have therefore undergone genetic modification without an external gene being introduced into their genome, while at the same time acquiring new characteristics.
Plant varieties derived from these techniques are already marketed in certain countries outside the European Union, and the diversity of NGT varieties could potentially increase, mainly due to the greater ease of use and low cost of CRISPR-Cas techniques. These offer a broader range of applications than those for the GMO plants currently authorised, including changes to plant yield or composition, tolerance to biotic or abiotic stress, or longer storage times.
Can current European regulations on GMOs be applied to NGT plants?
This context led the European Commission to launch a strategic initiative to adapt the current regulatory framework for GMOs to the specific characteristics of these NGT plants. As part of its work on biotechnologies and at the request of the Ministries of Agriculture and the Environment, ANSES conducted an expert appraisal to provide the authorities and stakeholders with insights on the challenges associated with the use of NGT plants in two areas:
- Methods for assessing the health and environmental risks of plants obtained through site-directed mutagenesis using the CRISPR-Cas system;
- The socio-economic challenges associated with NGT plants
On 5 July 2023, the European Commission proposed a regulation aimed at excluding certain NGT plants from European legislation on GMOs (Directive 2001/18/EC). They would be considered, on the basis of certain criteria, as equivalent to plants obtained using standard techniques. ANSES analysed these criteria with the support of its group of experts dedicated to biotechnologies. This analysis, published on 21 December 2023, was conducted in parallel with the expert appraisal on NGT plants published in March 2024, which had been initiated well before publication of the European Commission's proposal and was not intended to analyse it.
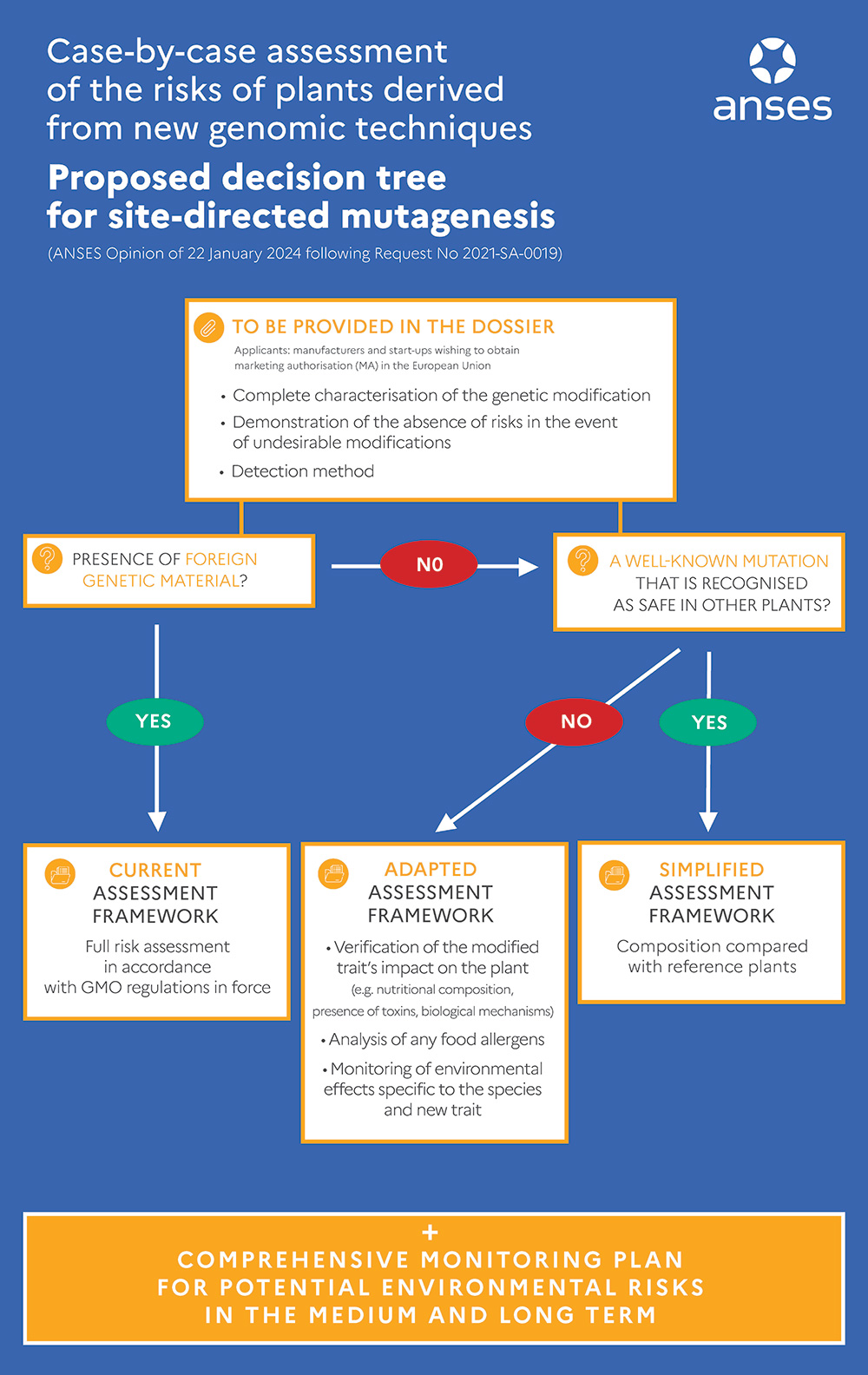
A case-by-case risk assessment tailored to NGT plants
ANSES's experts studied the risks associated with plants obtained using NGTs, particularly those resulting from site-directed mutagenesis using the CRISPR-Cas system, and their assessment methods. Following this analysis, the Agency deemed that the current framework for assessing health and environmental risks of genetically modified plants was only partially suitable for the assessment of these new plants.
The Agency therefore proposed a case-by-case assessment taking into account both the precision of the technique used and the characteristics of the plant obtained once the genome has been modified, while also considering all the potential toxicological, nutritional, agronomic and environmental consequences of the new characteristics. It developed a decision tree adapted to a graduated approach to risk. "To draw up this precise and comprehensive assessment framework, the experts mainly drew on data from the literature and case studies representative of the many possible applications. Depending on the case, the decision tree suggests maintaining the current assessment framework or conducting a simplified or adapted assessment. The choice of a simplified assessment is made by comparing the molecular, phytochemical, nutritional and agronomic characteristics of the plant obtained with the data available in the scientific literature," explains Youssef El Ouadrhiri, Head of the Biotechnologies Unit at ANSES.
If the mutation reproduces a modification of the genome observed in nature or already obtained by traditional techniques, and for which no risk has been identified, ANSES provides the option of simplifying the risk assessment framework.
For ANSES, some of the risks identified for NGTs are not radically different from those arising from transgenesis techniques, but the level of exposure to the plants obtained could be much higher considering the diversity of possible applications. The Agency therefore stressed the importance of post-marketing surveillance and recommended setting up a comprehensive mechanism to monitor NGT plants and derived products for health and environmental effects, as well as to observe changes in cultivation practices associated with these plants. Such surveillance would help fill the knowledge gap in the area of NGT plants and products while further safeguarding health and the environment with regard to their use.
Lastly, on the basis of the regulatory requirements that will ultimately be decided, ANSES called for common guidelines to be drawn up in order to limit differences in interpretation of risk assessment between the countries of the European Union.
What are the socio-economic challenges associated with the development of NGT plants?
ANSES's experts also analysed the potential socio-economic implications of different possible scenarios for regulatory changes relating to NGT plants. The expert appraisal therefore identified the areas of activity and stakeholders potentially concerned by NGT plants in four agricultural sectors (tomato, soft wheat, carrot and grapevine), representing the various possible applications of NGTs and different situations in terms of plant development, production, marketing and consumption in France.
Given the specific characteristics of each sector, it is likely that the introduction of NGT plants and products into the European Union would not affect them all in the same way. ANSES identified several major issues to be taken into account in the regulations, such as intellectual property associated with patents for plant breeding and concentration in the sector, as well as consumer information. Even though knowledge on these questions needs to be consolidated, ANSES recommended that the authorities be vigilant in limiting imbalances between stakeholders in terms of value-sharing and avoiding any abuses of dominant market positions. Expectations regarding traceability and detectability of NGTs may also have significant consequences for the sectors.
The Agency also underlined the wide variety of motivations that can lead to the development of breeding innovations: increasing the effectiveness or efficiency of agricultural and agro-industrial production, implementing product differentiation strategies and responding to health, environmental or societal challenges. All these motivations could be dealt with differently in future legislation and regulations. Support for public research would also be decisive in guaranteeing the capacity to develop innovations with a view to making the European agricultural and food system more sustainable.
The need for a debate that is as open and well-informed as possible
This expert appraisal work shows that the controversies surrounding NGT plants and products extend beyond the realm of safeguarding health and cover a much broader set of concerns relating to agricultural production models and the role of genomic technologies in an agro-ecological transition. "Amending regulations to take NGTs into account involves societal choices, because different economic and societal impacts are also at stake. This expert appraisal work by ANSES identifies all the questions that need to be asked in order to ensure that the debate is as open and well-informed as possible," explains Brice Laurent, Director of the Social Sciences, Economics & Society Department at ANSES.
Infography : Case-by-case assessment of the risks of plants derived from new genomic techniques (PDF)